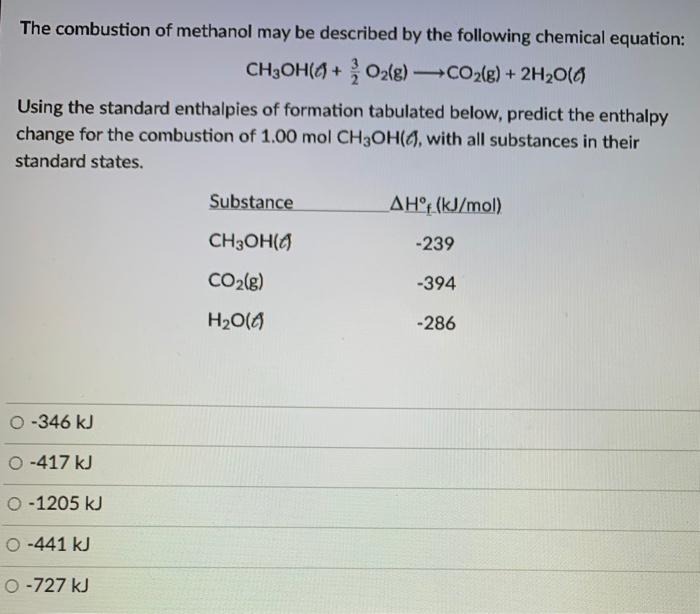
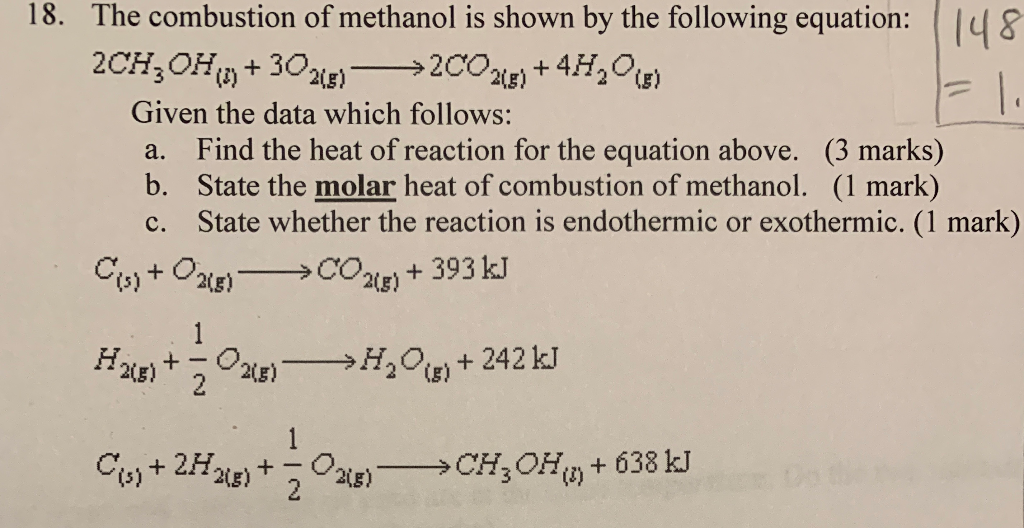
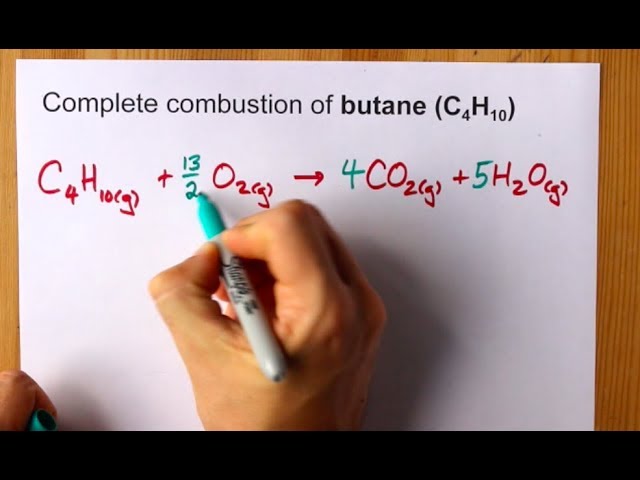
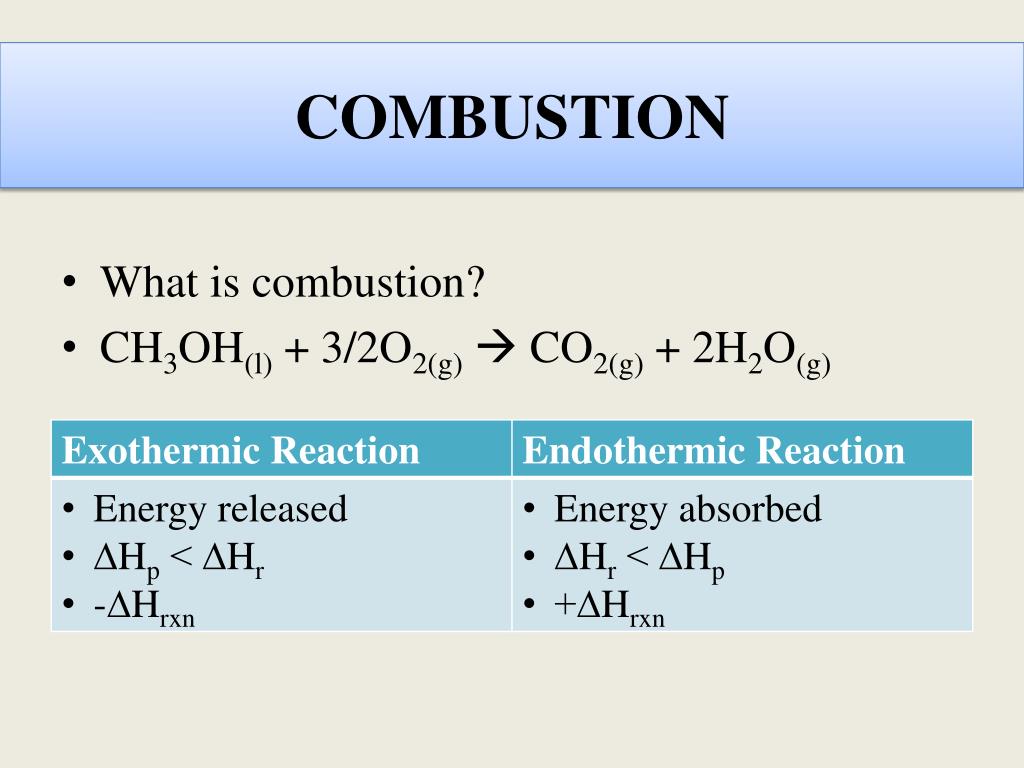
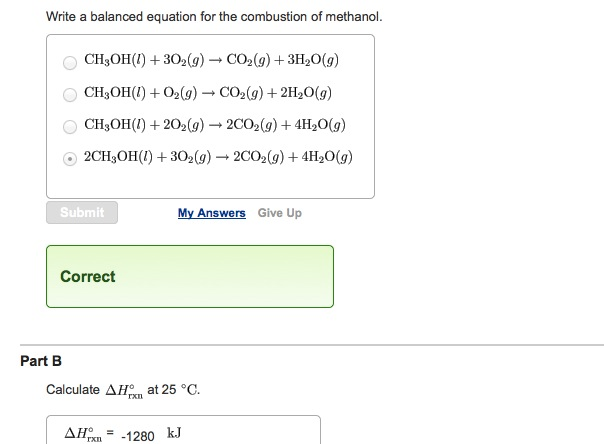
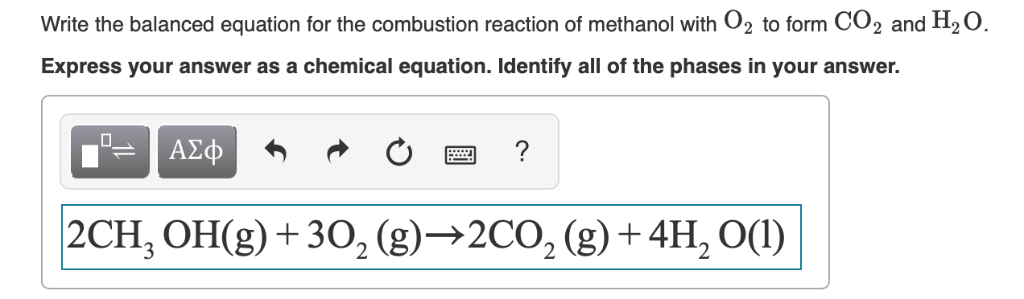
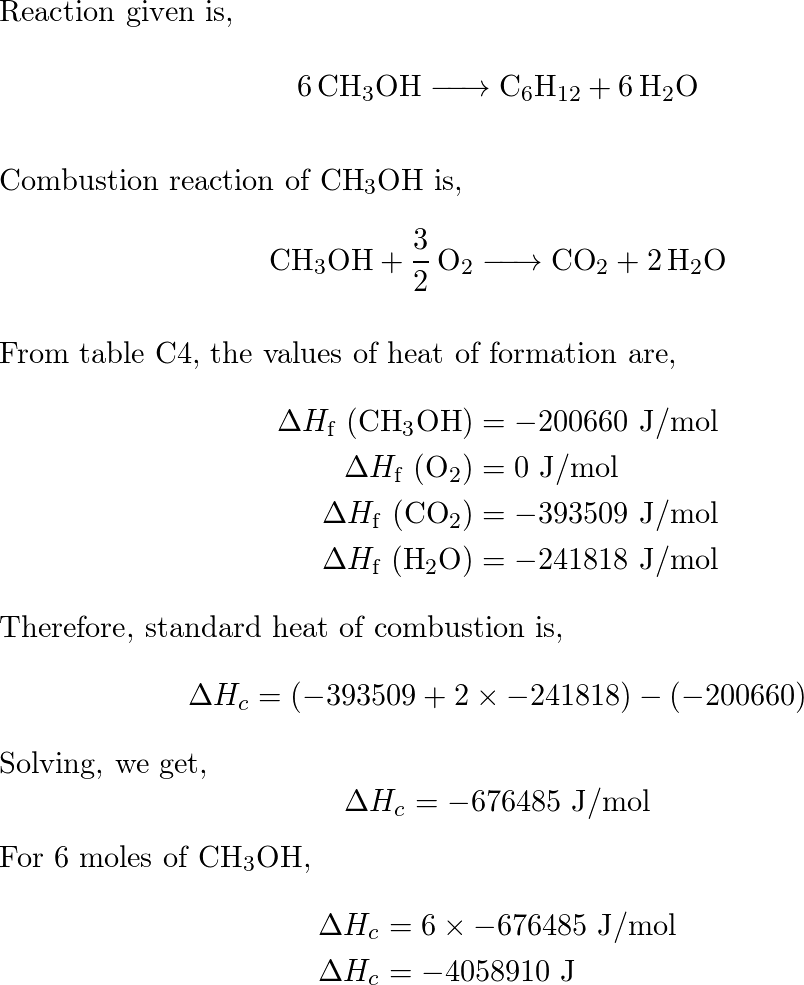
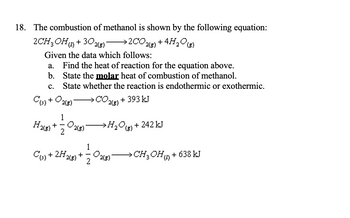
Calculate the enthalpy of combustion of methanol (CH3OH) given enthalpies of formation of CH3OH(1) CO2(g) - Sarthaks eConnect | Largest Online Education Community

SOLVED: Write an atom balanced chemical reaction equation for the complete combustion of methanol CH3OH with 30% excess air (21% oxygen and 79% nitrogen). Find the actual air fuel ratio (by mass) for methanol

SOLVED: According to the following combustion reaction, pure methanol liquid undergoes complete combustion with excess oxygen. If 64.05 liters, L of water vapor, H2O was recovered at STP, how many milliliters, mL

In a fuel cell, methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is: CH3OH(1) + 3/202(g) + CO2(g) + 2H2O(l) At 298 K, standard Gibb's

1. In a fuel cell, methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is CH,OH+ 30, CO2 + 2H20 (1) At 298K, standard Gibbs energies

SOLVED: Consider the reaction for the combustion of methanol (CH3OH): 2 CH3OH + 3 O2 â†' 2 CO2 + 4 H2O. What is the mass of oxygen (O2) that is required to

Schematic representation of a potential energy profile for methanol... | Download Scientific Diagram

NO-chemistry during methanol combustion from rich to lean conditions—exploring the fuel property influence mechanisms on different NO pathways - ScienceDirect

Question Video: Classifying a Named Reaction as Endothermic or Exothermic Based on the Reaction Enthalpy | Nagwa

In a fuel cell methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is CH3OH(l) + 32O2(g) → CO2(g) + 2H2O(l) At 298 K standard Gibb's